how to draw molecular orbital diagram of co
Watch the video solution for the question. The bonding MOs are the 2σ 1πx 1πy and 3σ which gives 2 2 2 2 8 bonding electrons.

Orbitals How To Rationalise With Mo Theory That Co Is A Two Electron Donor Through Carbon Chemistry Stack Exchange
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules.

. So again its drawn in the familiar pattern. The antibonding MO is the 2σ which gives 2. Its most important property is burning in air to give CO 2 in the combustion of fossil fuels.
Can be accommodated in the metal d orbitals. Explore bonding orbitals in other small molecules. CO molecule has 10 valence electronsfour from carbon atom 2s²2p² and six from oxygen atom 2s²2p⁴According to molecular orbital diagram molecular orbital configuration is given as σ2s² σ2s² πx² πy² σz² πx⁰ πy⁰ σz⁰ Thus bond order 12 823.
The MO diagram for CO is. So when youre drawing on a global diagram like this you have to draw it it should be schematically shown lower energy than the carbon. And this should make sense because no is isoelectronic with co which has a bond order of 3.
Draw the orbital diagram for the ion Co2. Hydrogen Fluorine Nitrogen Hydrogen Fluoride Carbon Monoxide Methane Ammonia Ethylene Acetylene Allene. BO 1 2 bonding e antibonding e 1 2 2 2 2 2 2 3.
Hence the bond order here is. 1s 2 2s 2 2p 2. AO2s AO2s σ2s σ 2s strong head-on overlap AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals.
Molecular orbitals in Carbon Monoxide CO. Hydrogen Fluorine Nitrogen. Find total valence electrons.
So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. Draw the orbital diagram for the ion Co2. Bond order N_b - N_a2 10 - 42 3.
CO is a very stable 10-valence-electron molecule isoelectronic with CN and with N 2 which has a slightly lower bond dissociation energy than CO. Draw the orbital diagram for ion Co 2. For virtually every covalent molecule that exists we can now draw the Lewis construction predict the electron-pair geometry predict the molecular geometry and come up close to predicting bond angles.
σ1s2 σ1s2 σ2s2 σ2s2 π2py2 π2pz2 π2px2. Draw the orbital diagram for ion Co 2. Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals.
Determine point group of molecule if linear use d2h and c2v instead of dh or cv 2. Electronic configuration of C atom1s22s22p2. Calculate their bond orders and give their magnetism diamagnetic or.
Next well see that symmetry will help us treat larger. Carbon monoxide strips oxygen off metal oxides reducing them to pure metal in high temperatures forming carbon. Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals.
Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. See how carbon monoxide acts as a ligand on transition metals. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each.
However one of the nearly important molecules we know the oxygen molecule O ii presents a problem with respect to its Lewis structure. Here we have a molecular orbital diagram for the CO molecule. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals.
Electronic configuration of CO molecule is. FracN_b-N_a2 frac10-42 3. Electronic configuration of CO molecule.
Molecular orbital diagram of Carbon monoxide molecule CO Electronic configuration of C atom. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. Bond order can be calculated by the formula.
Steps to draw hybrid orbital diagrams for a molecule step 1. Procedure for constructing molecular orbital diagrams based on hybrid orbitals 1. Explore bonding orbitals in other small molecules.
The antibonding MO is the 2σ which gives 2 antibonding electrons. The MO diagram for CO is. Use the buttons at the top of the tool to add orbitals in order of increasing energy starting at the bottom with the.
CO molecule has 10 valence electronsfour from carbon atom 2s²2p² and six from oxygen atom 2s²2p⁴According to molecular orbital diagram molecular orbital configuration is given as σ2s² σ2s² πx² πy² σz² πx⁰ πy⁰ σz⁰ Thus bond order 12 823. Not as much for the two P but the two P will also be at the lower energy the two P. Electronic configuration of O atom.
The molecular orbital diagram mot is useful to predict bond order bond strength bond energy. AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals. Electronic configuration of O atom.
Symmetry adapted linear combinations salcs we need salcs aka group orbitals to draw molecular orbital mo diagrams of polyatomic molecules. The formal bond order of CO is 3 from about one σ- bond and two π- bonds. In picture 2 we show the overlapping p orbitals which form the bond between the two fl.
σ 1s 2 σ 1s 2 σ 2s 2 σ 2s 2 π 2py 2 π 2pz 2 σ 2px 2. 1s 2 2s 2 2p 4. 12-12 This video describes the molecular orbital theory diagram of CO placing emphasis on how MO theory differs for homo and heteronuclear diatomics.
Molecular orbital energy level diagram of CO molecule can be given as. Bond order can be calculated by the formula. D0 ions d7 ions Fe1 Ru1 Co2.
Draw the mo for o 2. Draw the molecular orbital diagram of n2 also find its bond order.

Figure S6 Molecular Orbital Mo Diagram For The Valence Mos Of Ibr Download Scientific Diagram
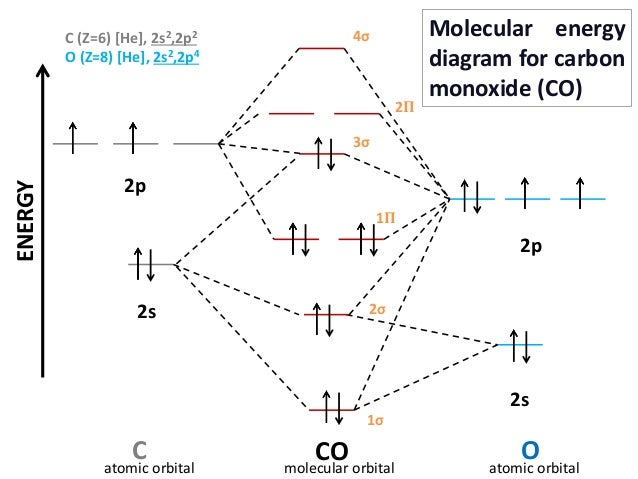
Molecular Orbital Diagram Of Co And No
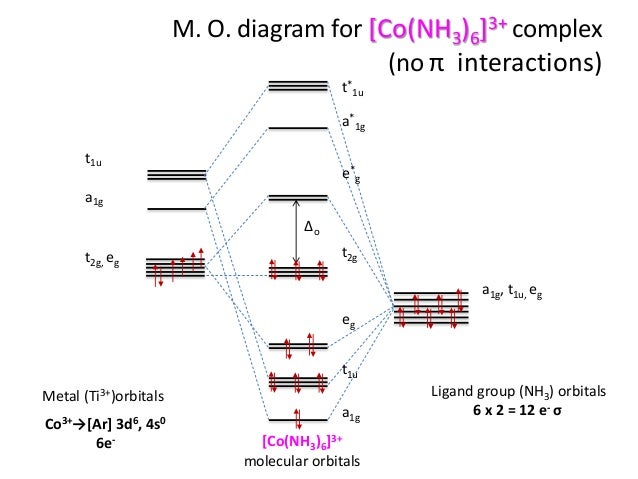
Molecular Orbitals Diagrams Of Co Nh3 6 3

Draw The Molecular Orbitals For Co In Order Of Energy And Fill Them With The Appropriate Number Of Electrons Label The Orbitals The Best You Can As Sigma Or Pi And As
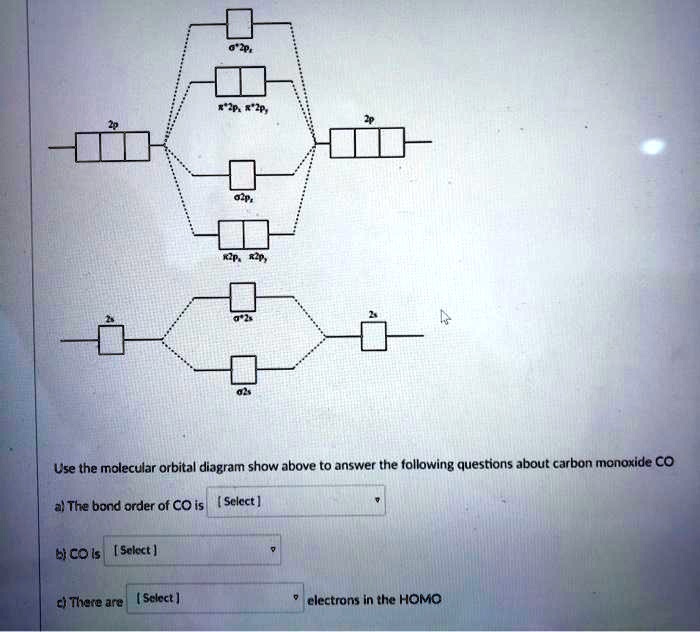
Solved Use The Molecular Orbital Diagram Show Above To Answer The Following Questions About Carbon Monoxide Co The Bond Order Of Co Is Sclect Bicois Select Ci There Are Sclect
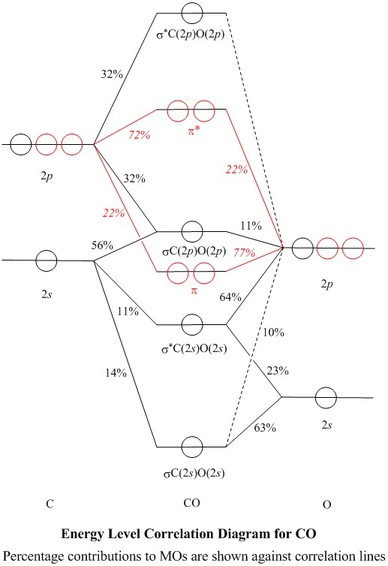
Molecular Orbitals For Carbon Monoxide

Introduction To Inorganic Chemistry Molecular Orbital Theory Wikibooks Open Books For An Open World
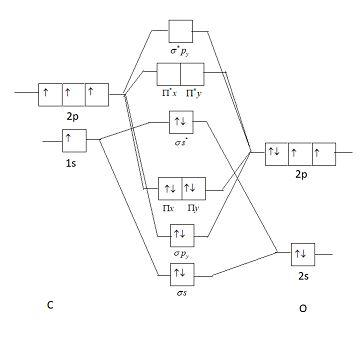
The Ground State Electronic Configuration Of Co Molecule Class 12 Chemistry Cbse

Molecular Orbital Diagram Of The Co Molecule Excluding 1s Atomic Download Scientific Diagram
Consider The Following Molecules No No And No Using The Molecular Orbital Theory How Do You Evaluate Them In Terms Of Bond Energy And Stability Quora

What Is The Molecular Orbital Energy Diagram Of Co Quora

Conceptual Mo Diagram Of Co Ii Iii L1 3 2 3 In High Spin And Download Scientific Diagram
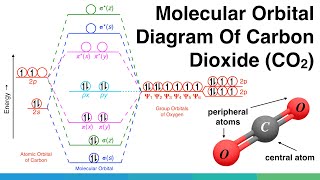
Molecular Orbital Diagram Of Polyatomic Co2 Molecules Chemical Bonding Molecular Structures Youtube

How Co Forms According To Molecular Orbital Theory Please Clarify The Concept Of Hybridization Involved In This According To Molecular Orbital Theory Quora

Draw The Molecular Orbital Diagram For Co Based On Your Diagram Why Does Co Always Bond Through The Carbon And Not The Oxygen Atom Study Com

